What Three Families Are Found in the Center Portion of the Periodic Table
The Periodic Police
- Page ID
- 621
The periodic law was developed independently by Dmitri Mendeleev and Lothar Meyer in 1869. Mendeleev created the showtime periodic table and was presently followed by Meyer. They both arranged the elements past their mass and proposed that certain backdrop periodically reoccur. Meyer formed his periodic constabulary based on the diminutive volume or tooth volume, which is the atomic mass divided by the density in solid course. Mendeleev's tabular array is noteworthy because information technology exhibits by and large authentic values for diminutive mass and information technology also contains blank spaces for unknown elements.
Introduction
In 1804 physicist John Dalton advanced the atomic theory of matter, helping scientists make up one's mind the mass of the known elements. Around the same time, two chemists Sir Humphry Davy and Michael Faraday developed electrochemistry which aided in the discovery of new elements. Past 1829, chemist Johann Wolfgang Doberiner observed that certain elements with like properties occur in group of three such as; chlorine, bromine, iodine; calcium, strontium, and barium; sulfur, selenium, tellurium; iron, cobalt, manganese. All the same, at the time of this discovery too few elements had been discovered and there was confusion between molecular weight and atomic weights; therefore, chemists never really understood the significance of Doberiner's triad.
In 1859 ii physicists Robert Willhem Bunsen and Gustav Robert Kirchoff discovered spectroscopy which immune for discovery of many new elements. This gave scientists the tools to reveal the relationships between elements. Thus in 1864, pharmacist John A. R Newland arranged the elements in increasing of diminutive weights. Explaining that a given set of properties reoccurs every eight identify, he named information technology the law of Octaves.
The Periodic Law
In 1869, Dmitri Mendeleev and Lothar Meyer individually came upward with their own periodic law "when the elements are arranged in order of increasing diminutive mass, certain sets of backdrop recur periodically." Meyer based his laws on the atomic volume (the diminutive mass of an element divided by the density of its solid grade), this property is called Molar volume.
\[\text{Diminutive (molar) book (cm}^3\text{/mol)} = \dfrac{\text{ molar mass (g/ mol)}}{\rho \text{ (cm}^3\text{/g)}}\]
Mendeleev'due south Periodic Table
Mendeleev's periodic tabular array is an arrangement of the elements that group similar elements together. He left blank spaces for the undiscovered elements (diminutive masses, chemical element: 44, scandium; 68, gallium; 72, germanium; & 100, technetium) then that certain elements can be grouped together. However, Mendeleev had non predicted the noble gases, so no spots were left for them.
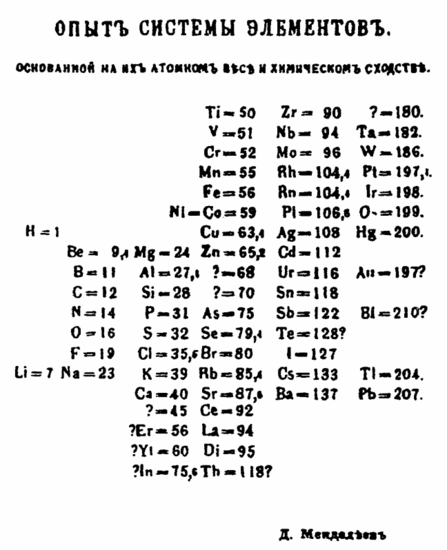
In Mendeleev's table, elements with like characteristics fall in vertical columns, chosen groups. Tooth volume increases from pinnacle to bottom of a groupiii
Example
The alkali metals (Mendeleev's group I) have loftier molar volumes and they also have depression melting points which decrease in the order:
Li (174 oC) > Na (97.8 oC) > K (63.seven oC) > Rb (38.ix oC) > Cs (28.5 oC)
Atomic Number as the Ground for the Periodic Police
Assuming in that location were errors in atomic masses, Mendeleev placed certain elements not in order of increasing atomic mass then that they could fit into the proper groups (similar elements take similar properties) of his periodic tabular array. An instance of this was with argon (diminutive mass 39.nine), which was put in front of potassium (diminutive mass 39.ane). Elements were placed into groups that expressed similar chemical behavior.
In 1913 Henry Thou.J. Moseley did researched the 10-Ray spectra of the elements and suggested that the energies of electron orbitals depend on the nuclear charge and the nuclear charges of atoms in the target, which is also known as anode, dictate the frequencies of emitted X-Rays. Moseley was able to tie the Ten-Ray frequencies to numbers equal to the nuclear charges, therefore showing the placement of the elements in Mendeleev's periodic table. The equation he used:
\[\nu = A(Z-b)^2\]
with
- \(\nu\): X-Ray frequency
- \(Z\): Atomic Number
- \(A\) and \(b\): constants
With Moseley's contribution the Periodic Law can exist restated:
Similar properties recur periodically when elements are arranged according to increasing atomic number."
Atomic numbers, not weights, determine the cistron of chemical properties. As mentioned earlier, argon weights more than potassium (39.9 vs. 39.one, respectively), yet argon is in front end of potassium. Thus, nosotros tin come across that elements are bundled based on their atomic number. The periodic law is plant to help determine many patterns of many unlike properties of elements; melting and boiling points, densities, electric conductivity, reactivity, acidic, basic, valance, polarity, and solubility.
The table below shows that elements increase from left to right appropriately to their atomic number. The vertical columns take similar properties within their group for example Lithium is like to sodium, beryllium is similar to magnesium, and and then on.
| Group | 1 | 2 | 13 | 14 | 15 | sixteen | 17 | 18 |
|---|---|---|---|---|---|---|---|---|
| Element | Li | Be | B | C | N | O | F | Ne |
| Atomic Number | three | four | five | 6 | 7 | eight | 9 | 10 |
| Atomic Mass | six.94 | nine.01 | 10.81 | 12.01 | 14.01 | fifteen.99 | 18.99 | 20.18 |
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| Atomic Number | 11 | 12 | 13 | 14 | fifteen | sixteen | 17 | 18 |
| Diminutive Mass | 22.99 | 24.31 | 26.98 | 20.09 | xxx.97 | 32.07 | 35.45 | 39.95 |
Elements in Group 1 (periodic tabular array) accept like chemical properties and are called brine metals. Elements in Group 2 have similar chemic properties, they are called the alkaline earth metals.
Curt form periodic table
The short form periodic table is a table where elements are arranged in seven rows, periods, with increasing atomic numbers from left to correct. There are 18 vertical columns known as groups. This table is based on Mendeleev's periodic tabular array and the periodic law.
Long grade Periodic Table
In the long course, each period correlates to the building up of electronic beat out; the first two groups (ane-2) (due south-block) and the last vi groups (13-18) (p-block) brand up the main-grouping elements and the groups (three-12) in between the south and p blocks are called the transition metals. Group 18 elements are called noble gases, and group 17 are called halogens. The f-cake elements, called inner transition metals, which are at the lesser of the periodic table (periods eight and 9); the fifteen elements after barium (atomic number 56) are called lanthanides and the 14 elements later on radium (diminutive number 88) are chosen actinides.
References
- Petrucci, Ralph H., William S. Harwood, F. G. Herring, and Jeffrey D. Madura. General Chemical science: Principles and Mod Applications. 9th ed. Upper Saddle River: Pearson Education, Inc., 2007.
- Sisler, Harry H. Electronic construction, properties, and the periodic law. New york; Reinhold publishing corporation, 1963.
- Petrucci, Ralph H., Carey Bissonnette, F. Thousand. Herring, and Jeffrey D. Madura. General Chemical science: Principles and Mod Applications. Custom Edition for CHEM 2. Pearson Learning Solutions, 2010.
- Mendeleev's 1869 Periodic Table. In Wikimedia Eatables. Retrieved 4 December 2010, from commons.wikimedia.org/wiki/Fi...odic_table.png.
- Periodic Tabular array of Elements. In Wikimedia Commons. Retrieved 4 December 2010, from eatables.wikimedia.org/wiki/Pe...le_of_Elements.
Problems
one ) The periodic police force states that
- similar properties recur periodically when elements are bundled according to increasing atomic number
- similar properties recur periodically when elements are arranged according to increasing atomic weight
- similar properties are everywhere on the periodic table
- elements in the same period have same characteristics
2) Which element is most like to Sodium
- Potassium
- Aluminum
- Oxygen
- Calcium
3) According to the periodic law, would argon be in front of potassium or subsequently? Explain why.
4) Which element is most similar to Calcium?
- Carbon
- Oxygen
- Strontium
- Iodine
five) Who were the two chemists that came up with the periodic law?
- John Dalton and Michael Faraday
- Dmitri Mendeleev and Lothar Meyer
- Michael Faraday and Lothar Meyer
- John Dalton and Dmitri Mendeleev
Answers
- A
- A
- Argon would in front of potassium because the periodic constabulary states that the periodic table increases from left to right based on atomic number not atomic weights
- C
- B
Source: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/The_Periodic_Law
0 Response to "What Three Families Are Found in the Center Portion of the Periodic Table"
إرسال تعليق